Abstract
Background: Varnimcabtagene autoleucel (IMN-003A) is an autologous second-generation CAR T-cell product with a 4-1BB co-stimulatory domain and a non-FMC63 based murine single chain variable fragment targeting CD19 (A3B1 binder), manufactured in India. As previously published, pre-clinical studies and phase 1 clinical trial in Spain, varnimcabtagene autoleucel (IMN-003A / ARI-0001) has demonstrated potent in vitro, in vivo and clinical activity. Here, we report the first safety and efficacy results of the IMAGINE phase 2 clinical trial for patients in India with relapsed/refractory B cell malignancies (CTRI/2022/03/041162).
Methods: Patients (pts) aged 3 to 45 years (B-ALL) and ≥ 18 years (B-NHL) with relapsed and/or refractory B cell malignancies (RR BCM) were eligible for this study if they had measurable disease, as assessed by lymphoid blasts (B-ALL) or metabolic tumour bulk (B-NHL), received ≥1 prior regimen, refractory to the last line of treatment with good performance status (ECOG 0 to 1). Bridging therapy was allowed after apheresis. Cyclophosphamide (300 mg/m2) and fludarabine (30 mg/m2) on days -5 to -3 were used as lymphodepletion regimen. The target dose was 1x106/kg CAR+ cells (B-ALL) and 5x106/kg CAR+ cells (B-NHL) (overall range 0.1x106 to 5x106) and was administered in a fractionated manner (10%/30%/60%), with at least 24h between infusions on days 0, +1, +2. Primary objectives were overall response rate (ORR: CR + CRi in B-ALL and CR + PR in B-NHL) at day +90 after first infusion, and occurrence of adverse events including cytokine release syndrome (CRS) and/or Immune Effector Cell Associated Neurotoxicity Syndrome (ICANS). Response was assessed as per NCCN (B-ALL) and IWG (B-NHL) criteria; bone marrow minimal residual disease (MRD) for B-ALL was analyzed by flow cytometry at 10-4 sensitivity and PET-CT for B-NHL. Adverse events (AEs) were graded using CTCAE v5.0. CRS and ICANS were graded according to the American Society for Transplantation and Cellular Therapy (ASTCT) criteria.
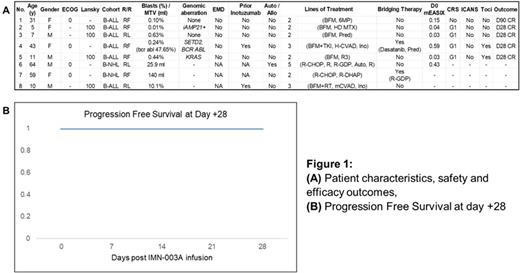
Results: As of data cut-off, eight pts (median age 21 yrs, range 5 - 64) with RR BCM (n=6 B-ALL; n=2 B-NHL) have been included in the trial. 6 pts received IMN-003A cells (intention-to-treat population), of which 2 (33.3%) received bridging therapy. The main characteristics of these pts and previous treatments are described in Figure 1A. Median CAR-T cell manufacturing time was 11 days (range 10-18) with 100% manufacturing success.
Median follow-up after IMN-003A administration was 50 days (range 5 - 117). Of the 5 evaluable patients, ORR at day +28 (n=4) and day +90 (n=1) was 100%. Median time to first response was 28 days. All pts were in CR at data cut-off.
Of 5 MRD-evaluable pts at day +28 (n=4) and +90 (n=1), 100% were MRD-negative. Median progression-free survival (PFS, Figure 1B) and overall survival (OS) were not reached.
AESIs reported in 8 pts were CRS (Grade [G] 1 50%; G3+ 0%; overall 50%), neutropenia (G3+ 50%; overall 62.5%), anemia (G3+ 0%; overall 62.5%), and thrombocytopenia (G3+ 0%; overall 62.5%). Median onset and duration of CRS was on Day +10 and 2 days respectively. No CAR-T cell-related neurotoxicity (ICANS) was reported. Tocilizumab was administered in 33.3% (n=2/6) (for persistent grade 1 CRS). No mortality has been reported in this study till date.
IMN-003A cells demonstrated peak expansion on day 10 (range 10 - 14 days). 80% (5/6 evaluable pts) had measurable CAR+ T cells in peripheral blood on day +28. Updated results will be presented in the meeting.
Conclusion: Varnimcabtagene autoleucel (IMN-003A) is a First-In-India Industry CD19-directed CAR-T Cell Therapy for RR BCM that has demonstrated excellent outcome in a clinical trial, with deep and durable responses and a favorable safety profile, including absence of neurotoxicity. Varnimcabtagene autoleucel offers a significant benefit over standard treatment options for patients in India for RR BCM.
Disclosures
Joseph:Immuneel Therapeutics Private Limited: Current Employment. Jakka:Immuneel Therapeutics Private Limited: Current Employment. Dhar:Immuneel Therapeutics Private Limited: Current Employment. Kumar MG:Immuneel Therapeutics Private Limited: Current Employment. Palanisamy:Immuneel Therapeutics Private Limited: Current Employment. Elluru:Immuneel Therapeutics Private Limited: Current Employment. Akheel:Immuneel Therapeutics Private Limited: Current Employment. Anand:Immuneel Therapeutics Private Limited: Current Employment, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees. Kamat:Immuneel Therapeutics Private Limited: Current Employment, Current holder of stock options in a privately-held company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal